
We specialise in providing comprehensive CSA training and consulting services to regulated industries to ensure your software meets the highest standards of quality and compliance.
The FDA released a draft guidance document "Computer Software Assurance for Manufacturing, Operations and Quality System Software" during 2022. It introduces a new approach to CSV called 'Computer Software Assurance' (CSA), which shakes up the conventional approach of Computer System Validation (CSV). CSA encourages a more thoughtful and risk-oriented mindset, focusing on what really matters - patient safety whilst maintaining product quality and safeguarding data integrity.
CSV, as it stands today, is a documentation-heavy exercise which is done at the expense of critical thinking and testing. CSA brings about a paradigm shift by encouraging critical thinking over documentation. By leveraging the tenets of CSA, companies can execute more testing with less documentation.
Determine:
- the Impact of the system (Direct/Indirect/None)
- the Category (Non-configured/Configured/Custom)
- the Type of testing (Ad Hoc/Unscripted/Scripted)
Perform scripted testing for direct impact systems and URS items that are customisable.
Ensure risk is aligned appropriately to requirements
When final, this guidance will supplement FDA's guidance, "General Principles of Software Validation" ("Software Validation guidance") except this guidance will supersede Section 6 ("Validation of Automated Process Equipment and Quality System Software") of the Software Validation guidance.
GAMP 5 2nd Edition is aligned with CSA guidance, in regard to patient safety, product quality and data integrity. The main issues highlighted in this edition surround innovation, critical thinking, agile methodologies and IT service management. The guidance shows a clear evolution from Computer Systems Validation (CSV) to Computer Software Assurance (CSA)
It is important to note that “Unscripted” does not mean “Undocumented.”An unscripted test case defines the test objective, but does not include detailed test steps.
Limiting required documentation significantly reduces test script / tester errors, while it increases detection of functional irregularities that may be encountered in the live environment.
The shift to Computer Software Assurance is part of a broader trend being driven by industry bodies such as the FDA and the ISPE. It’s aimed at replacing compliance based CSV activity with measured, sensible, quality-based CSA actions, for example, replacing manual paper-based systems with electronic systems.
The Agile approach is focused on delivering quality and value during product development of customised applications at speed. It uses an incremental product configuration that promotes technical innovation and flexibility.
The planning, specification, configuration, verification and reporting are not linear, but incremental, iterative, and exploratory. This permits developers to meet compliance and demonstrate fitness for the intended use with less burdensome than with the linear model (V-model).
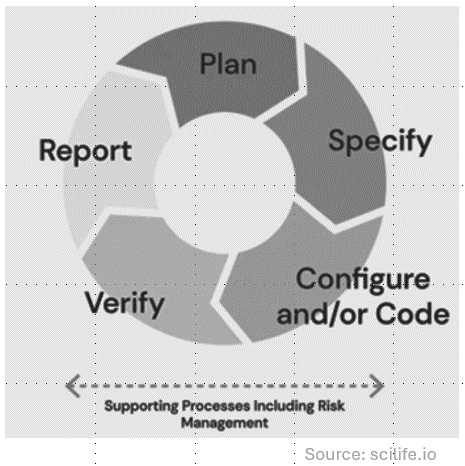
CSA is not new, it is based on existing regulations. However, the shift in focus in relation to documentation and testing requires expert intervention. We can provide guidance to:
- Build trust in the risk-based approach to CSA
- Redirecting resources to the right activities
- Find the balance between testing and validation
CSA is about critical thinking and collaborative teamwork.

We provide expert advice on Computer Software Assurance and regulatory compliance.
We also provide bespoke training programs to educate your team on best practices in software assurance
Based in Cork, serving Ireland and the EU